J.J Thomson Atomic model
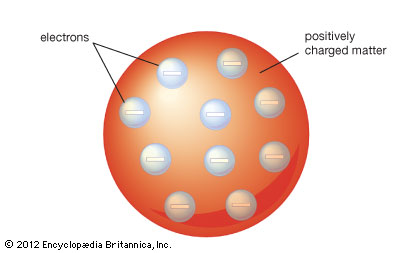 From this he discovered that no matter what metal
the electrodes were made up of or what type of gas was in the tube, the
mass to charge ratio was unchanged. He also deducted that there were two
parts of atom held together by electrostatic attraction. A heavier
part, responsible for the majority of mass of the atom and a negatively
charged ‘corpuscle’. This meant that Dalton’s model of an atom was incorrect, since the atom was not the smallest possible particle.
From this he discovered that no matter what metal
the electrodes were made up of or what type of gas was in the tube, the
mass to charge ratio was unchanged. He also deducted that there were two
parts of atom held together by electrostatic attraction. A heavier
part, responsible for the majority of mass of the atom and a negatively
charged ‘corpuscle’. This meant that Dalton’s model of an atom was incorrect, since the atom was not the smallest possible particle.
Comentarios
Publicar un comentario