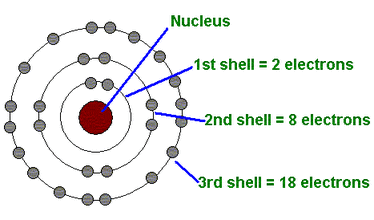
Bohr Atomic model
Bohr agreed with
Rutherford’s proposal that in the atom the electrons revolve around a
central positively charged nucleus that is responsible for most of the
weight of the atom. But from his special evidence, he concluded that
electrons are found at only certain distances from the nucleus, and have
particular values of energy. ohr’s atomic theory also helped to explain the
spectra. He explained the discrete lines on the hydrogen spectra by
proposing if a hydrogen atom was given energy, an electron can jump to a
higher shell, away from the nucleus. However they will always return
back to their ground state as the atom will be unstable.
Comentarios
Publicar un comentario